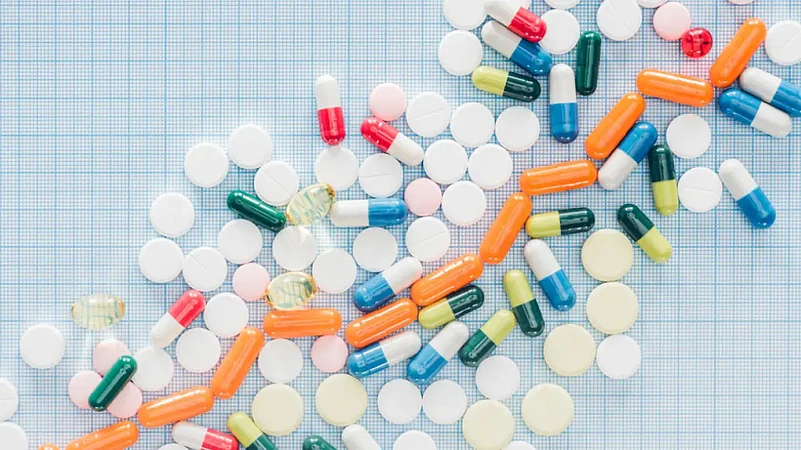
Last year, the alleged deaths of children in African country of Gambia because of an Indian syrup put limelight on India’s pharmaceutical company, which is otherwise hailed as the 'pharmacy of the world'.
India is the world's leading manufacturer of vaccines and is also the largest producer of generic drugs — cheaper pharmaceutical products made to match brand-name drugs in quality and performance as well as safety.
According to the reports, over 20 per cent of global supply of generic drugs already comes from India. With rising exports, India's pharmaceutical industry is expected to only rise.
However, the massive pharmaceutical market also carries more risk of tainted and counterfeit medicine that throws challenges to test the strength of India's regulators. Of late, scandals involving Indian pharmaceutical companies have been reported abroad. Here we look at some major alleged pharma scandals.
Cough syrup in Gambia
Last year, there were deaths of 66 children in Gambia allegedly from an India-made syrup. The incident put the spotlight on India's drug manufacturers following reports that the children's condition allegedly deteriorated after they were given Indian-made cough syrup.
The cough syrup was manufactured by India's Maiden Pharmaceuticals and exported to the African nation under four different brands.
World Health Organization (WHO) had issued a stern warning. The health agency had stated: "The products were ‘contaminated’. It had said the cough syrup triggered symptoms including ‘abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury which may lead to death'."
The Indian company in its response had said that the “diligent protocols” were followed in the production process of the syrup. It had also said that it was "shocked" and "deeply saddened" by the incident in Gambia.
The incident also prompted India to form a committee to investigate the case. The findings of the probe are yet to come out.
Uzbekistan Case:
In December 2022, 18 deaths occurred in Central Asia’s Uzbekistan. The country’s Health Ministry said 18 of 21 children with acute respiratory diseases allegedly died after ingesting Dok-1 Max syrup.
The drug was manufactured by Marion Biotech Pvt Ltd, a company based in Noida, Uttar Pradesh. The firm Quaramax Medical LLC had imported the syrup. Marion Biotech had a licence from the Food Safety and Drug Administration department in Uttar Pradesh to manufacture the cough syrup and tablets for export.
India said on December 29 in 2022 that the Central Drugs Standard Control Organisation (CDSCO) and the FSDA of UP had “immediately on receipt of the information” conducted a joint inspection of the Marion Biotech facility. Samples were sent to the Regional Drug Testing Laboratory in Chandigarh. The results are awaited.
US Case:
Few days back, a Tamil Nadu-based pharmaceutical company voluntarily recalled its eye drop from the US. It was after the eye drop drug was allegedly related to a drug resistant infection. It has been linked to 55 alleged incidents of infections, loss of vision, and even a death due to the infection entering the bloodstream in US.
The alleged infection was linked to multi-state cluster of a bacteria called pseudomonas aeruginosa that was resistant to third-line antibiotic called Carbapenem.
US Food and Drug Administration urged people to stop selling and using the eye drop. The eye drop was distributed by ErziCare and Delsam Pharma.
Multiple drug recalls in United States
The Indian drug-maker Lupin Limited also some days back recalled 5,720 tubes of a skin ointment over quality issues, according to US Food and Drug Administration (FDA).
The Lupin's US arm Lupin Pharmaceuticals Inc has recalled the tubes of Clobetasol propionate Cream from the US market. The medicine was recalled after the US FDA found it to be of alleged poor quality. The ointment was used in the treatment of various skin conditions, such as dermatitis, eczema, and psoriasis.
The ointment recalled from US was manufactured at Lupin's Pithampur plant in Madhya Pradesh.
The formal reason for the recall was following the FDA observation, who found it: "subpotent Drug: Low assay result observed during long-term stability testing".
Lupin initiated the Class III nationwide (US) recall of the affected lot on January 23 this year. As per the FDA, a class III recall is initiated in a "situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences".
The US generic drug market was estimated to be around USD 115.2 billion in 2019. It is the largest market for pharmaceutical products. Indian medicines have a long history of quality issues, either not having enough drugs or having impurities that can cause adverse conditions.
This was not the first time that Lupin came on FDA's radar. The company has recalled products at least four times over the past year over quality issues and failing regulatory tests.
In January 2023, Lupin recalled 16,056 bottles of Rifampin Capsules used in the treatment of all forms of tuberculosis. The recall was observed after impurities were detected and the drug failed stability test.
In December 2022, Lupin recalled blood pressure medicine Quinapril over impurities in the tablets. The prolonged use of the impurity detected can cause cancer, WebMD quoted FDA as saying.
The Hill reported, "The tablets were contaminated by substances known as nitrosamines, which the FDA reports are commonly found in food and water.These impurities are found in meats, dairy products and vegetables, and can increase a person’s risk of developing cancer when faced with prolonged exposure, according to the FDA."
In September 2022, Lupin recalled 7,872 bottles of same Rifampin Capsules which were also recalled last month. The recall was for manufacturing lapses.
In January 2022, Lupin recalled 50,832 bottles of anti-bacterial medicine Gatifloxacin Ophthalmic Solution. It's used in eye treatment. The recall was ordered after failing stability tests.
In January 2022, it was also reported that Lupin was recalling 23,965 bottles of Oxycodone Hydrochloride tablets. The tablets are used for the treatment of moderate to severe pain.
Other cases of Indian pharma companies:
In 2016, two Indian pharmaceutical companies were charged for exporting counterfeit diabetes drugs.
Food and Drug Administration (FDA) investigated Pharmaceutical Products of India and Wanbury and found the firms had a manufacturing and export deal with which they were illegally rebranding and exporting the diabetic drug metformin hydrochloride to Bangladesh, Brazil, Mexico and Pakistan.
The reports mentioned that the illegal activity had been going on for several years.
In 2013, Ranbaxy Laboratories pleaded guilty to charges over the manufacture and distribution of adulterated drugs.
The company agreed to pay 500 million dollars under a settlement agreement with the US Department of Justice to settle civil and criminal cases relating to sub-standard drugs and false data given to the US health authorities.
During Covid-19 pandemic in 2022, fake vials of remdesivir, an antiviral drug used to treat the virus, were sold in bulk at astronomical prices and even exported.
20% Indian drugs fail tests: Report
A report by United States Trade Representative (USTR) found that 20 per cent of all pharmaceutical products sold on the Indian market are counterfeit.
The reports quoting official figures reveal that between 2007 and 2020, more than 7,500 drugs sampled in the country’s just three states out of 28 states and three union territories had failed quality tests.
In 2018, the Central Drug Standard Control Organization identified about 4.5 per cent of all generic drugs in the country’s market to be substandard.
Reports said one-fourth of the 12,000 manufacturing units in India were found to fulfill with the WHO's good manufacturing practices, the obligatory quality regulations that pharmaceutical companies must adhere to.