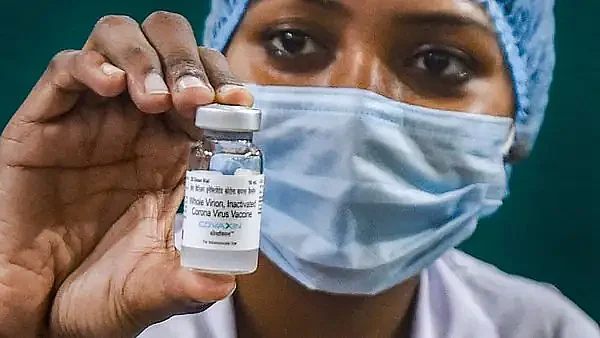
Amid the ongoing debate over the UK-based Covid-19 vaccine manufacturer AstraZeneca's recent admission that its Covid-19 shot, labeled as 'Covishield' in India, could cause 'rare' side effects, Bharat Biotech, the Indian vaccine maker that developed Covaxin, on Thursday took it to X to highlight its safety record.
The Covid-19 vaccine manufactured by AstraZeneca was sold globally under the brand names Covishield and Vaxzevria among others.
What all did Bharat Biotech say?
In its official statement released on its X handle, Bharat Biotech said that Covaxin was developed with a single-minded focus on safety first, followed by efficacy.
Furthermore, Bharat Biotech also highlighted that Covaxin was the only Covid-19 vaccine in the government's Covid-19 immunisation programme to have conducted efficacy trials in India.
"Covaxin was evaluated in more than 27,000 subjects as part of its licensure process. It was licensed under restricted use in clinical trial mode, where detailed safety reporting was carried out for several hundred thousand subjects," Bharat Biotech said.
"Safety of Covaxin was also evaluated by the Ministry of Health, Government of India. Ongoing safety monitoring (pharmacovigilance) was continued throughout the product life cycle of Covaxin," it stated.
The company said that studies and follow-up activities demonstrated its "excellent safety record" for Covaxin and that there were no reports of vaccine-associated incidents, including blood clots, Thrombocytopenia, pericarditis and myocarditis.
"As seasoned innovators and product developers, the Bharat Biotech team was well aware that, while the efficacy of Covid-19 vaccines may be short-lived, the impact on patient safety could last a lifetime. Hence, safety is the primary focus for all our vaccines," it further said.
'Very rare side effect': AstraZeneca's recent admission
Recently, for the very first time, UK-based pharma company AstraZeneca admitted in court documents that in 'very rare cases' Covishield could cause a blood clot-related side effect.
In India, the AstraZeneca vaccine was manufactured by the Serum Institute of India (SII) and was marketed as Covishield.
AstraZeneca's admission on court paper came as the pharmaceutical company was facing a class-action lawsuit that alleged serious injuries and deaths resulting from its vaccine which was manufactured in collaboration with the University of Oxford.
As per reports, the lawsuit was filed by Jamie Scott, who suffered a permanent brain injury after receiving the AstraZeneca vaccine in April 2021.